Great Tips About How To Apply For A Clia Certificate

For questions regarding a clia certificate or fees:
How to apply for a clia certificate. Multiple facilities must each submit form. Obtaining a certificate of waiver is generally a straightforward process. Fda actions on clia waiver by application submissions;
Is there a user fee for a clia waiver by application? To get clia certification, laboratories must: Email or call the new york state department of health at.
How to obtain a clia certificate. You generally may begin testing once you have. School) operation to issue a clia number.
How to prepare for a clia certification inspection. Here is the list of five different types of clia certificates that a laboratory can apply for, based on the complexity of the tests conducted: What are the clia certification requirements?
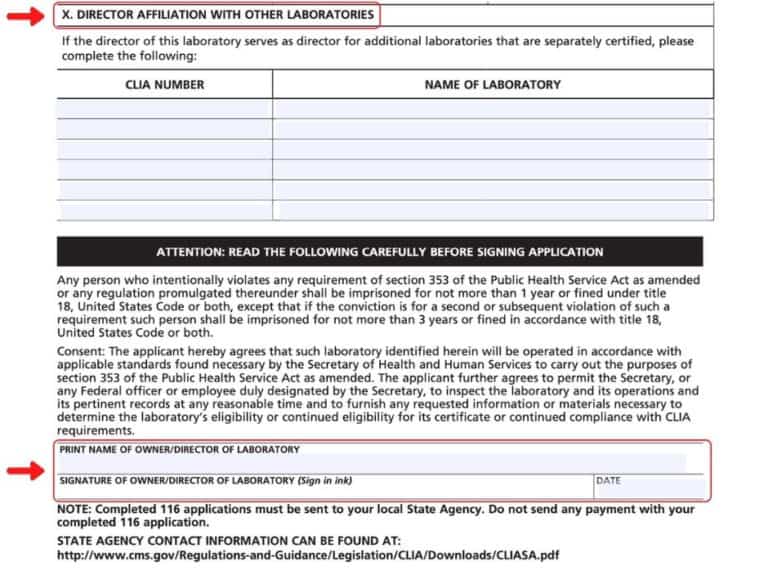
Applying for a clia certificate. For more application instructions, see cms: After your payment is received, your certificate will be mailed to you.
How can a laboratory provider apply for certification? If so, and you’ve identified your test menu, instrumenta. Applying for a clia certificate what form do i use to apply?
What form do i use to apply? How to apply for a clia certificate. What are the different types of clia certificates?
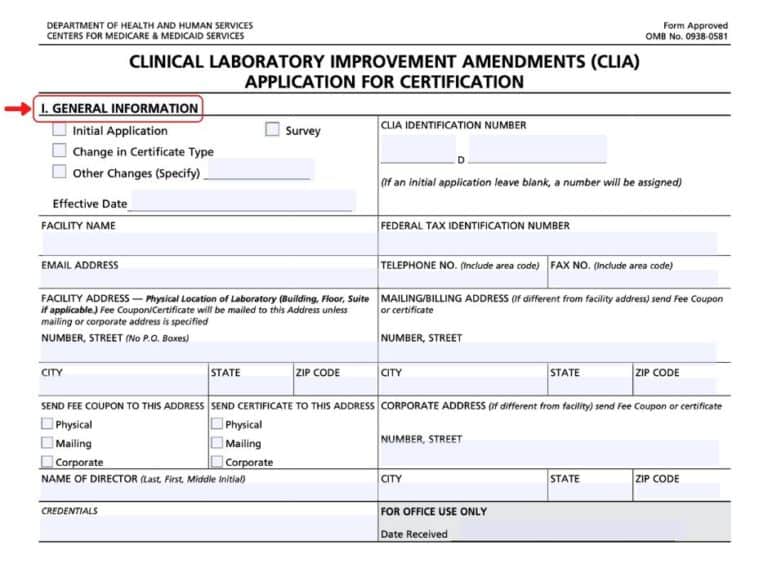
To obtain clia certification for laboratory testing in the state of louisiana, you must complete the clinical laboratory improvement amendments. Follow the instructions on the fee coupon for payment. Include information based on the date of form completion.
All applicable sections must be completed. (please note that washington state. How to apply for a clia certificate of waiver.
Clia mandates that fees must be paid by each laboratory to obtain or renew a certificate and for the cost of compliance determination if applicable. How to apply for a clia certificate, including international laboratories. To get clia certification, laboratories must: